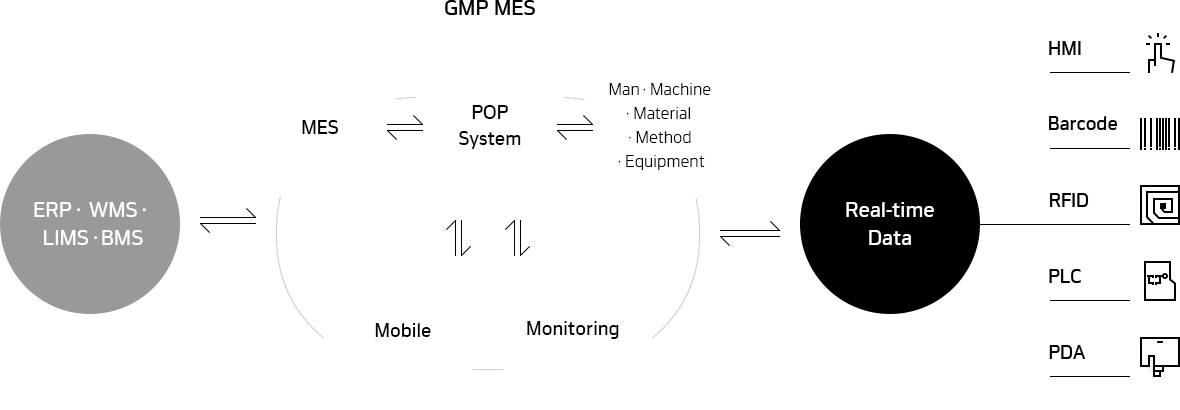
MES
We connect all devices in your plant to provide process optimization
with manufacturing-related information in real time.
GMP MES
Good Manufacturing Practice Manufacturing Execution System
(GMP MES)
GMP MES is an execution system for good manufacturing practice. As a specialized MES solution against GMP-based regulation laws for pharmaceutical/cosmetic manufacturing, it contributes to your business’s operation by providing flexible actions against regulations on GMP and FDA21 CFR Part 11, satisfying the conditions for CSV (Computer System Validation), and strengthening your business’s competitiveness in both manufacturing and product quality.
-
GMP MES
- System management
- Master Data management
- Manufacturing records management
- Work order management
- Manufacturing process management
- Quality management
- Logistics management
- Traceability management
- Facility maintenance management
- Approval process management
- Annual quality evaluation management
-
POP System
- Electronic SOP management (weighing, manufacturing, packaging)
- Production performance management
- Personnel / facility management
- Raw material / material management
- Semi-finished product / work-in-process
- Carrying Container Management
- Notice
- Equipment connection S/W
-
Mobile
- Raw material / material management
- Raw Material / Warehouse Management
- Semi-finished product management
- Semi-finished product Warehouse Management
-
Monitoring
- Urgent reports on manufacturing
- Urgent reports on quality
- Facility operation
- Personnel in manufacturing
- Announcements
Features
-
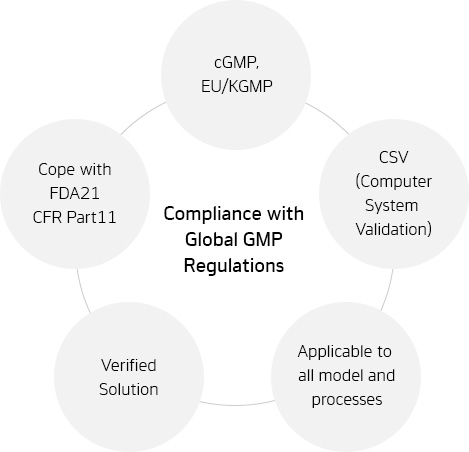
Compliance with Global GMP
Reliable management against globalization/advancement of GMP
-
Fulfills Requirements from FDA 21 CFR Part 11/CSV
Cope with CSV(Computer system validation)
-
Application to Various Product Groups and All Processes
Tool support for creation and simulation of electronic records and electronic signatures
Implementation to all manufacturing processes from loading raw material to packaging
Flexibility against definition and changes in work order
-
Consistency in Data and Flexible Reponses in Field
Automatic collection of recipe/parameter data from manufacturing facilities
Prompt response to expanding or redesigning processes/facilities
- Contact Us
- ManagerKun Woo Kim
- kku2148@hankookn.com
- 031 - 5178 - 8375010 - 8260 - 4918
- Contact Us
- ManagerYun Jin Oh
- yunjin456@hankookn.com
- 031 - 5178 - 8376 010 - 8260 - 4492
